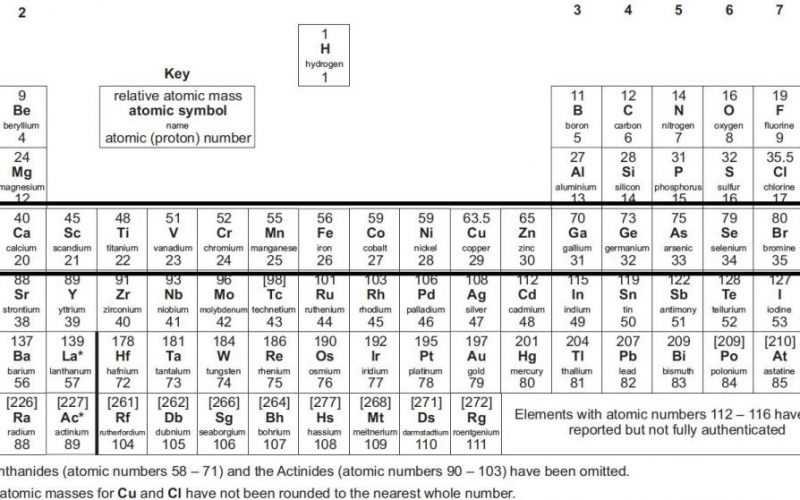
One of the most essential charts in chemistry is thought to be the periodic table. For more than a century, Chemists all across the world have been using this chart, which arranges all of the elements based on their characteristics.
In 1869, Dmitri Mendeleev created the periodic table, and since then, it has undergone updates to take into account new elemental discoveries and studies.
Even today, GCSE and A-level chemistry courses still use the periodic table, which has been around for more than 150 years.
In this piece, we’ll be explaining everything you need to know about the GCSE periodic table.
Table of contents
- What Is The Periodic Table & Why Is It Important?
- How Are Elements Arranged On The Periodic Table?
- Information From The Periodic Table?
- Do You Need To Know The GCSE Periodic Table
- Can You Take Periodic Table with You on the GCSE Chemistry Exam?
- Atomic Numbers Relation To Element Properties
- Frequently Asked Questions
- Conclusion
- References
- Recommendations
What Is The Periodic Table & Why Is It Important?
The Periodic Table is like a map of all the elements in the universe. It helps organize these elements to make it easier to understand and predict their behaviors.
Every element comprises tiny particles called atoms, and each atom has its unique properties. Some elements are shiny and conduct electricity well, like copper. Others might be gases that we breathe, like oxygen.
But there are so many elements out there—over a hundred of them! So, scientists needed a way to make sense of all this diversity.
The periodic table contains all known elements and are arranged according to their atomic number, starting with hydrogen, which makes up 90% of all atoms, and ending with Oganesson, a recently found element.
Atomic number indicates the number of protons in each atom of that element. The positively charged protons that comprise an atom’s nucleus are surrounded by electrons, which revolve around it.
The Table is organized into rows called “periods” and columns called “groups.” Each element has its spot on the Table, and its location tells us a lot about it.
The rows help us see patterns in how the elements’ properties change as we go from left to right. And the columns show us how elements with similar properties are grouped.
But why is the Periodic Table so important? Well, it’s like a treasure map for scientists. By studying the Table, they can predict how different elements will react with each other and even discover new elements that haven’t been found yet.
Also read: What Are GCSEs Equivalent In Scotland?
How Are Elements Arranged On The Periodic Table?
The arrangement of elements on the Periodic Table is not arbitrary; it is based on their properties and characteristics.
This arrangement allows scientists to understand and predict the behavior of elements and their compounds. The Periodic Table is organized into periods (rows) and groups (columns), each with significance.
Periods (Rows):
Periods are horizontal rows on the Periodic Table, numbered from 1 to 7. Each period represents a different energy level or electron shell that the elements’ outer electrons occupy.
As you move from left to right across a period, the atomic number (number of protons) increases by one, indicating the addition of one proton and one electron to each successive element.
The properties of elements change as you move across a period. Elements on the left side of a period are typically metals, while those on the right are non-metals.
The transition metals are found in the middle of the Periodic Table and have unique properties that make them useful in various applications.
Groups (Columns):
Groups, also known as families, are vertical columns on the Periodic Table, numbered from 1 to 18.
Elements within the same group share similar chemical properties due to their electron configurations. Each group has a representative element that showcases the group’s characteristics.
The elements in a group have the same number of valence electrons (electrons in the outermost shell), which determines their chemical reactivity.
For example, elements in Group 1 (the alkali metals) all have one valence electron, making them highly reactive with water and air.
Group 18 elements, known as noble gases, have full valence electron shells and are chemically inert.
Blocks:
The Periodic Table is often divided into blocks based on the type of subshell being filled with electrons.
The four blocks are s-block, p-block, d-block, and f-block. Each block has a distinct shape on the table corresponding to a specific energy level and electron arrangement.
Periodic Trends:
The arrangement of elements on the Periodic Table reveals various trends in properties as you move across periods and down groups. Some essential trends include atomic radius (size of atoms), ionization energy (energy required to remove an electron), electron affinity (tendency to gain electrons), and electronegativity (ability to attract electrons in a chemical bond).
Also read: What to Do if You Fail Your GCSEs in 2024 | Expert Advice for 2024
Information From The Periodic Table?
Each element’s box on the Periodic Table contains a wealth of information that provides insights into its properties, characteristics, and atomic structure. Let’s break down the critical components of an element’s box and what they signify:
- Element Symbol: The element’s chemical symbol, usually a one- or two-letter abbreviation, uniquely identifies the element. For example, “H” represents hydrogen, and “O” represents oxygen.
- Atomic Number (Z): The atomic number represents the number of protons in the nucleus of an atom of that element. It is a fundamental property that distinguishes one element from another. Elements are arranged on the Periodic Table in order of increasing atomic number.
- Atomic Mass: The atomic mass, also known as atomic weight, is the average mass of that element’s isotopes, considering their abundances. It is typically expressed in atomic mass units (AMU).
- Electron Configuration: This information shows how the electrons are arranged in the energy levels or electron shells around the nucleus. It provides insights into an element’s chemical behavior and reactivity.
- Period Number: The period number corresponds to the energy level or electron shell where the valence electrons are found. Elements in the same period share similar energy levels for their outer electrons.
- Group Number: The group number indicates the number of valence electrons an element has. Elements in the same group typically have similar chemical properties due to their electron configurations.
- Element Category: The element’s placement within the s-block, p-block, d-block, or f-block of the Periodic Table indicates the type of subshell being filled with electrons. This affects the element’s electron arrangement and chemical properties.
- State at Room Temperature: Some Periodic Tables provide information about whether the element is a solid, liquid, or gas at room temperature.
Also read: Top 10 Hardest GCSE Maths Questions in 2024
Do You Need To Know The GCSE Periodic Table
As a student who wants to take the GCSE Chemistry examination, it is a must that you know the periodic table.
When we say you need to know the periodic table, we don’t mean you should know it by heart. We simply mean you should be able to know and interpret every information on it.
You may wonder why it is important. Let’s find out below.
Foundation of Chemistry
The GCSE Periodic Table is the foundational framework of chemistry. It organizes the building blocks of matter, the elements, systematically and meaningfully.
Understanding the Periodic Table is essential for grasping more advanced concepts in chemistry, as it provides a basis for understanding how elements interact, form compounds, and exhibit various properties.
Predicting Element Behavior
Knowledge of the GCSE Periodic Table allows you to predict the behavior of elements based on their position in the table.
Elements within the same group often share similar chemical properties due to their identical valence electron configurations.
This predictive ability becomes crucial when studying chemical reactions and understanding why certain elements react in specific ways.
Identifying Trends and Patterns
The Periodic Table reveals numerous trends and patterns in element properties as you move across periods and down groups. For example, atomic radius, ionization energy, and electronegativity exhibit predictable changes based on an element’s position.
Understanding these trends helps explain why certain elements exhibit particular characteristics and how they might interact with other elements.
Nomenclature and Symbols
The Periodic Table introduces you to the symbols and names of the elements. These symbols are used universally in scientific communication and are essential for understanding chemical formulas, equations, and reactions. Identifying elements by their symbols is a fundamental skill in chemistry.
Applications in Daily Life
Chemistry impacts our daily lives in various ways, from the materials we use to the medicines we take. Understanding the Periodic Table helps you comprehend the properties and uses of different elements.
For example, knowing sodium (Na) is a highly reactive metal can help you understand its role in table salt (sodium chloride).
Educational and Career Pursuits
If you’re considering a career in a scientific field, such as chemistry, medicine, engineering, or environmental science, understanding the Periodic Table is not just important—it’s essential.
A solid grasp of the Periodic Table can make higher-level chemistry courses and specialized fields more accessible.
Critical Thinking and Problem-Solving
Learning about the Periodic Table cultivates critical thinking skills. As you study the arrangement of elements and the patterns they exhibit, you’ll develop an ability to analyze and solve problems related to chemical interactions and reactions.
Also read: How To Revise For GCSE English Language?
Can You Take Periodic Table with You on the GCSE Chemistry Exam?
In most cases, GCSE Chemistry exams have specific guidelines and rules regarding the use of the reference materials, including the Periodic Table.
These rules can vary depending on the exam board, the level of the exam (Foundation or Higher), and the specific exam paper. Here’s a detailed overview:
Foundation Level Exams
At the Foundation level, GCSE Chemistry exams are designed for students or grades 1 to 5. These exams typically assume a foundational understanding of chemistry concepts and may not permit the use of external reference materials, including the Periodic Table.
This is because the questions are tailored to the content covered in the syllabus, and candidates are expected to demonstrate their understanding without relying on additional resources.
Higher Level Exams
At the Higher level, GCSE Chemistry exams are intended for students aiming for grades 4 to 9. These exams require a deeper understanding of chemistry concepts and may involve more complex problem-solving.
Some exam boards might allow students to bring a copy of the Periodic Table as part of their permitted resources. However, even at this level, the specific rules can vary.
Allowed Formulas and Information
In cases where the Periodic Table is allowed, it might be provided within the exam paper itself, or students might be allowed to bring in a specific version provided by the exam board.
Additionally, students might be allowed to use certain specific formulas or information that is with the exam.
It’s important to note that any information provided by the exam board, including the Periodic Table, might be tailored to the exam and include every detail in a standard Periodic Table.
Checking Exam Regulations
Before taking your GCSE Chemistry exam, it’s crucial to carefully review the exam regulations provided by your school, teacher, or the exam board itself.
These regulations will outline what reference materials, if any, you are allowed to bring into the exam. If Periodic Table is permitted, ensure that you are familiar with the version that will be provided or that you are allowed to bring.
Also read: Top 10 Best GCSE Maths Revision Websites | 2024 Ranking
Atomic Numbers Relation To Element Properties
The atomic number of an element is a fundamental property unique in identifying an element. It is denoted by the symbol “Z” and represents the number of protons in the nucleus of an atom of that element.
In a neutral atom, the atomic number also corresponds to the number of electrons surrounding the nucleus. The atomic number defines an element and its place on the Periodic Table.
Relationship to Element Properties:
- Element Identity: The atomic number is the critical factor distinguishing one element. No two elements have the same atomic number. For example, carbon has an atomic number of 6, while oxygen has an atomic number of 8. This distinct atomic number ensures that elements have unique properties.
- Periodic Trends: The atomic number is crucial in determining periodic trends patterns in element properties that recur across the Periodic Table. As you move across a period (from left to right), the atomic number increases, resulting in changes in properties like atomic radius, ionization energy, and electronegativity.
- Valence Electrons and Reactivity: The atomic number also governs the number of valence electrons, which are electrons in the outermost energy level. Valence electrons largely determine an element’s reactivity and ability to form chemical bonds. Elements in the same group (column) have the same number of valence electrons and, therefore, exhibit similar chemical behaviors.
- Chemical Families: Elements with similar atomic numbers tend to belong to the same chemical family or group. For instance, the alkali metals in Group 1 have one valence electron and share similar chemical properties. The noble gases in Group 18 have full valence electron shells and are chemically inert.
- Ionization Energy: The atomic number affects ionization energy, which is required to remove an electron from an atom. Generally, ionization energy increases as you move across a period due to the increased nuclear charge. Elements with higher atomic numbers tend to have higher ionization energies.
- Electronegativity: This is the tendency of an atom to attract electrons in a chemical bond, tends to increase as you move across a period. Elements with higher atomic numbers usually have higher electronegativities.
Frequently Asked Questions
The number at the top of the box is the atomic number (Z), representing the number of protons in the nucleus. The element symbol is a one- or two-letter abbreviation for the element’s name. The atomic mass or atomic weight is usually provided below the element symbol.
Elements in the same group have the same valence electrons, leading to similar chemical behaviors. For instance, Group 1 elements (alkali metals) have one valence electron and are highly reactive, while Group 18 elements (noble gases) have full valence electron shells and are inert.
Periodic trends are patterns in element properties that recur as you move across periods and down groups.
Exam rules vary, but some exams may allow you to use a provided version of the Periodic Table. You must check your exam regulations to see if you can bring it and what information it contains
Most elements fit within the Periodic Table’s arrangement, but some very heavy elements, such as those with atomic numbers greater than 118, may not yet have stable forms and are less understood.
Conclusion
The atomic number and chemical characteristics are the organizing principles for the GCSE periodic table.
It includes all of the known elements, along with their electron configurations, atomic masses, and atomic numbers. It also displays their chemical characteristics, such as their positions in groups or periods, oxidation state, and valency.
Understanding the interactions between various elements, which ones are most likely to create compounds, and how those compounds might behave in practical scenarios are all made easier with the aid of the periodic table.
When you go for the GCSE Chemistry exam, they will give you a copy of the periodic table for your use.
References
- online-learning-college.com – The periodic table
- studymind.co.uk – The Current Periodic Table (GCSE Chemistry)
- edumentors.co.uk – GCSE Periodic Table Explained 2024